David Cooper
David Cooper from Uniqure spoke about the current status and future plan for the first gene therapy trial for HD involving an AAV5 expressing a microRNA targeting HTT human exon-1 sequences, termed AMT-130. David described the preclinical work conducted to support the clinical trial data, in work that is largely published. Ongoing studies in mini pigs support the activity by 36 months. The safety studies in transgenic mini pigs are five years long. However, AMT-130 does not target pig HTT, so these studies do not address loss of function consequences due to HTT lowering (not described by David but important to keep in mind).
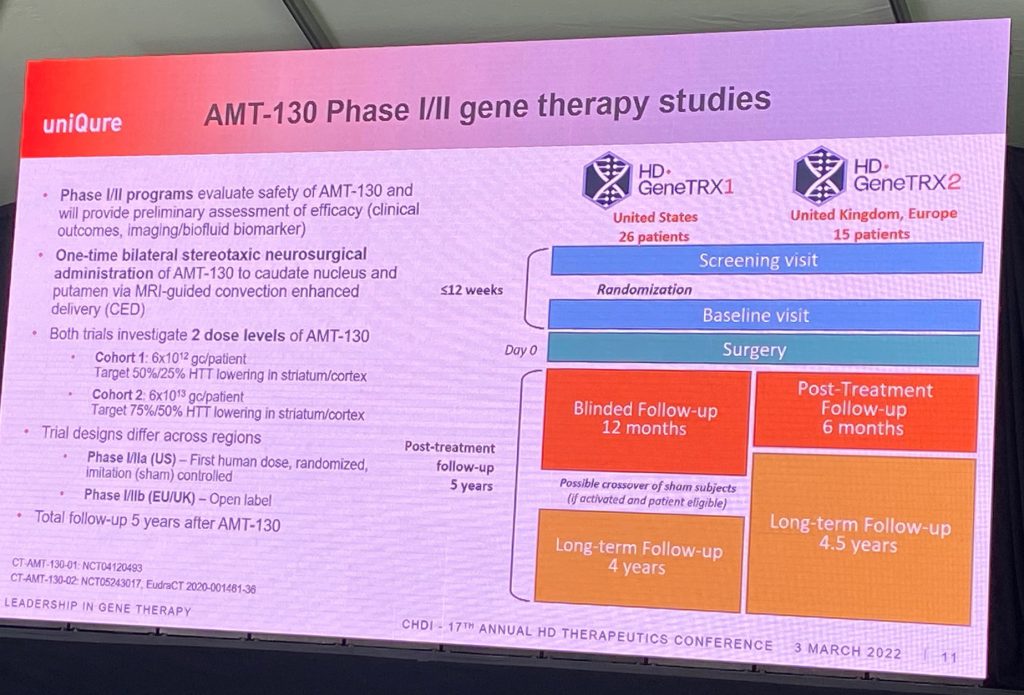
David then described the ongoing HD-GeneTRX-1 and -2 trials (see below), with a 5-year follow-up period. In the ongoing US-based GeneTRX-1 study, n=6 patients are in the low-dose group, and n=10 with high dose and n=10 sham; in the European HD-GeneTRX-2 study, n=15 patients expected to enroll (n=6 low and n=9 high dose). Biomarkers include mHTT, total HTT, NEFL, imaging, and other exploratory markers.
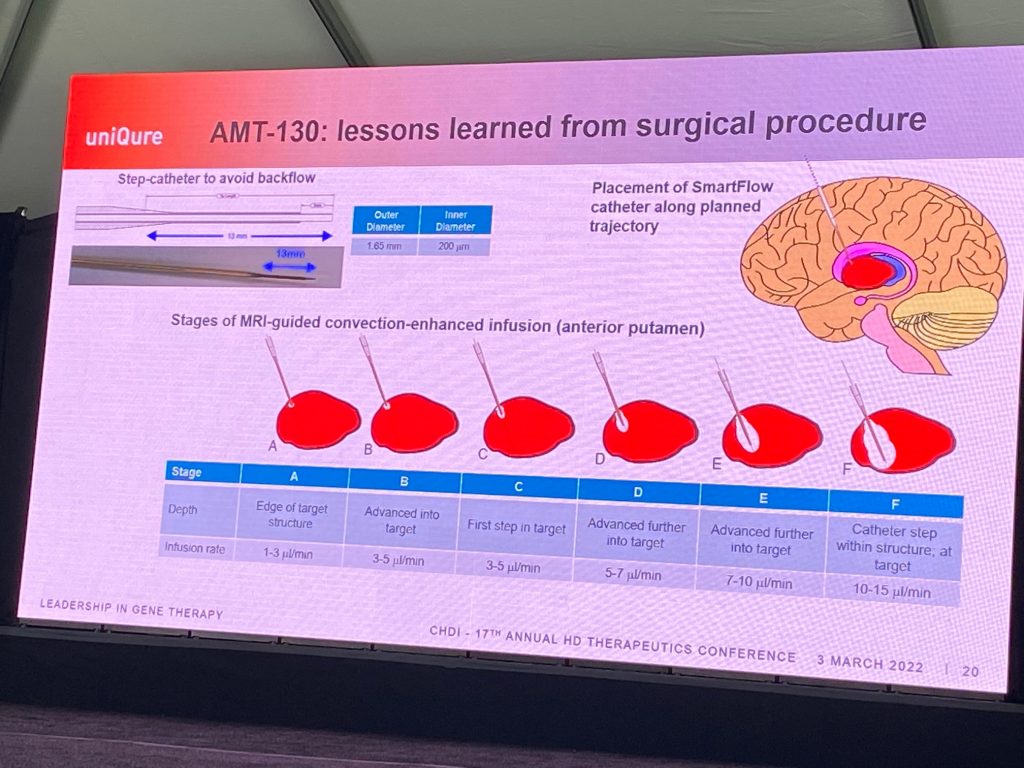
David described learnings from the surgical procure in HD (6 infusions, 3 sets of 2), which includes infusion into head of the caudate, a first for HD as well. confirmed they reached their goal of covering 65-70% volume of caudate and putamen. David described the method of conversion enhanced delivery over the 90′ period of the drug administration. Surgeons discuss each case and learn from each others’ experience during surgery, to ensure consistent techniques.
So far, treatments have been well tolerated (first 4 individuals, after 12m) and had no serious adverse events. NFEL levels were increased after surgeries but returned to baseline; the data on mHTT and tHTT is inconclusive. The European trial arm has begun in Poland. A key challenge now is obtaining biomarker data that theotherapy is working at the target. Another challenge is the administration procedure, which needs to be improved: trajectory (explore single frontal trajectory to putamen, which would reduce number of catheters), catheter implantation (ClearPoint SmartFlow), MRI-guided CED infusion to maximize distribution and control infusions in real-time. They are considering the use of robotics to accelerate time to place the catheters in the 6 locations.