Jan Vesper, Dusseldorf University
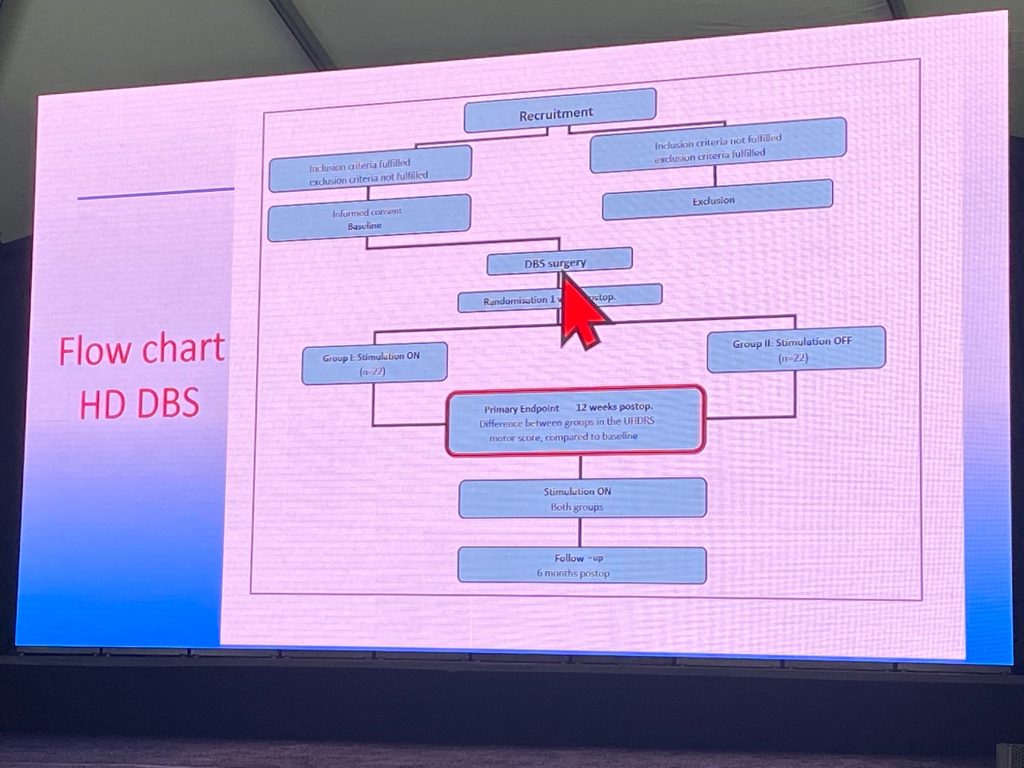
Jan Vesper spoke on the status update of the recently completed European randomized-controlled DBS trial of the globus pallidus (GP) for the treatment of HD (HD-DBS study). Prior work done in a pilot study in 2014 showed a significant reduction in chorea scores of the UHDRS. This prior small trial (n=6) showed that the GPe/GPi border was the region that best responded to DBS (less cell bodies, more axonal fibers). The HD DBS trial is a 6 month trial – 3 months blinded, 3 months open follow-up, in 4 countries (Germany, Switzerland, France and Austria). A sample size of n=50 randomized subjects, n=80 patients to be screened. Primary endpoint is UHDRS-TMS 12 weeks after surgery in the on-group. Secondary endpoints include many such as qMotor and dyskinesia scores, Quality of Life scores, etc. Inclusion criteria was very strict, with cognitively intact function and significant chorea despite best medical treatment. Cortical atrophy grade 3 were excluded- a scoring system was generated since German authorities required it, to ensure trajectory could take place during electrode placement. The scoring system is 0 (no atrophy), 1 , 2 and 3 (severe atrophy), and has been published
Microelectrode recordings are conducted during surgery and implantation to ensure the right placement. Patient is awake enough during anesthesia to obtain good recordings of the GPe/Gpi. The GPe was highly hyperactive as reported before in HD, and the GPi was hypoactive. The silent border in between was easily identifiable and where the 4-contact final electrode is placed. Lower contact stimulation in the GPi and Upper contact in the GPe using the Activa PC system from Medtronic.
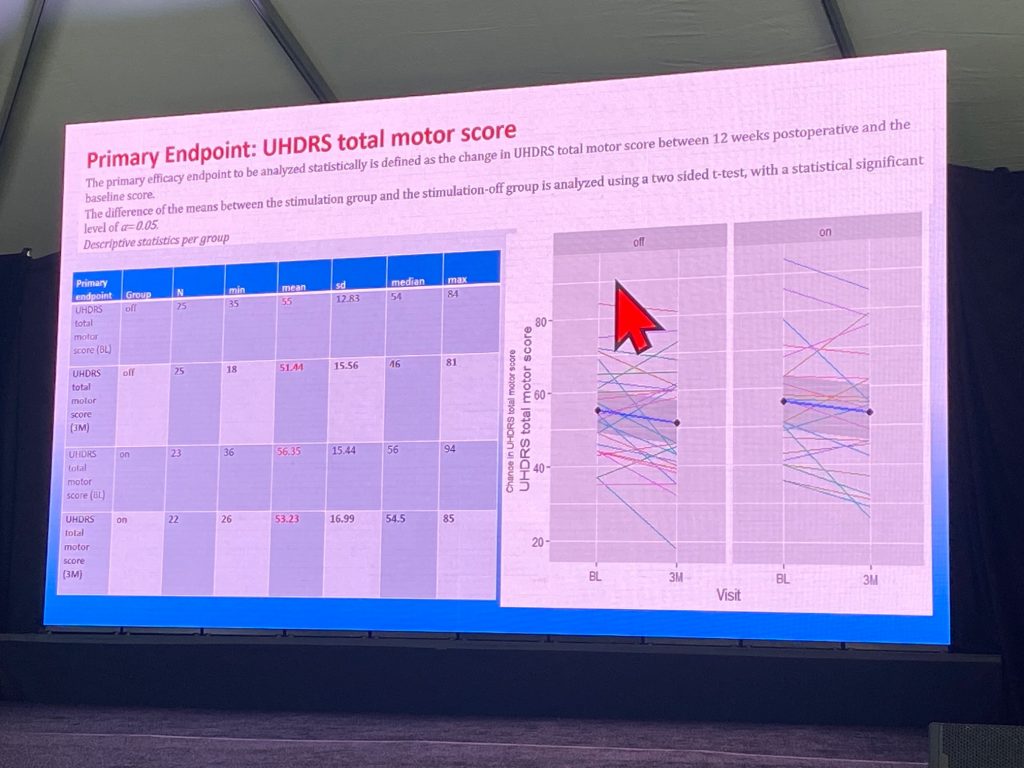
The trial included 56 patients at screening, n=48 randomized, with n=25 off group and n=23 in the on group. Population was balanced between the two groups at baseline and for all secondary endpoint items. Preliminary analysis (one week old!) shows the following: the TMS did not improve and the two groups were not significantly different. The only secondary endpoint analyzed is the UHDRS chorea sub score. this also showed no difference at all in the -on vs the -off groups. These results are very disappointing indeed.