Dr. José Lucas from the Center for Molecular Biology Severo Ochoa in Madrid spoke about this recent work in the role of mHTT in altered splicing and adenylation of mRNAs in HD. Jose started by describing his pioneering work in tau splicing alterations in the HD brain. He also described splicing changes in TAF1 protein, which leads to striatal degeneration and dyskinesias in a rare genetic disorder. He postulates that mHTT leads to changes in SRSF6 with multiple effects, one to modulate Tau splicing, and sec and, to a TAF1 loss of function. Because of these independent observations, his lab pursued a deeper unbiased analysis of RNA binding proteins and global missplicing events occurring in HD. He identified 342 genes misspliced in human and rodent samples, including several genes known to lead to degeneration when mutated. He then focused on altered splicing factors that might explain the phenotypes observed, such as TIA1, hNRPC, U2AF2, and RBFOX, the latter one being a key factor that is down regulated in HD. They created a transgenic mouse over-expressing RBFOX1 and crossed them to the R6/1 mouse model, leading to an attenuation of disease. He referred to work done in 2011 by Dr. Nancy Bonini and focused on a set of genes known to moderate RNA toxicity in flies, including musclebound and the orb2 (CPEB4 is the mammalian ortholog), a gene that regulates polyadenylation. CPEB provides also regulation of translation at synapses in a temporal manner. Jose then showed that CPEB1 and -4 are altered in HD, and determined that 20% of the transcripts have changes in adenylation, including SLC19A3, a transporter for thiamine in the brain endothelium. Deficiencies in SLC19A3 can be restored by administration of biotin, presumably via an effect on SLC19A3 levels. Therefore, if his hypothesis is correct, then HD patients should have less thiamine concentration in CSF and in brain parenchyma. A final set of studies showed that thiamine and biotin supplementation therapy can restore behavioral changes in R6/2 and Q175 models. A patent has been filed, and a small clinical study has been planned (called the HUNTIAM study) to evaluate safety of this supplementation strategy and the doses that work in mice are very high and they showed some toxicity in the rodent studies. Additional work to extend the diminished levels of thiamine in CSF is bering explored in a longitudinal study with the Sant Pau hospital in Barcelona.
Dr. MICHAEL RAPE
Dr. Michael Rape from Berkeley University and the Howard Hughes Medical Institute spoke about the mechanisms that degrade the HTT protein via the proteasome, a critical step in regulating the expression level of the protein, and an important area for therapeutic development. Key challenges for this area have been the identification of ubiquitin-ligase complexes that can modify HTT and target it for degradation, and the specific lysine amino acid residues that get modified and that regulate its half-life. He then describes the recent unpublished work that has led him to identify the E3 ligase that works to modify HTT directly and degrade it. His team has reconstituted HTT ubiquitination in vitro, opening the way for identification of HTT modified sites, and of a screening system to identify molecular glues – small molecules that promote the interaction between HTT and the identified E3 ligase.

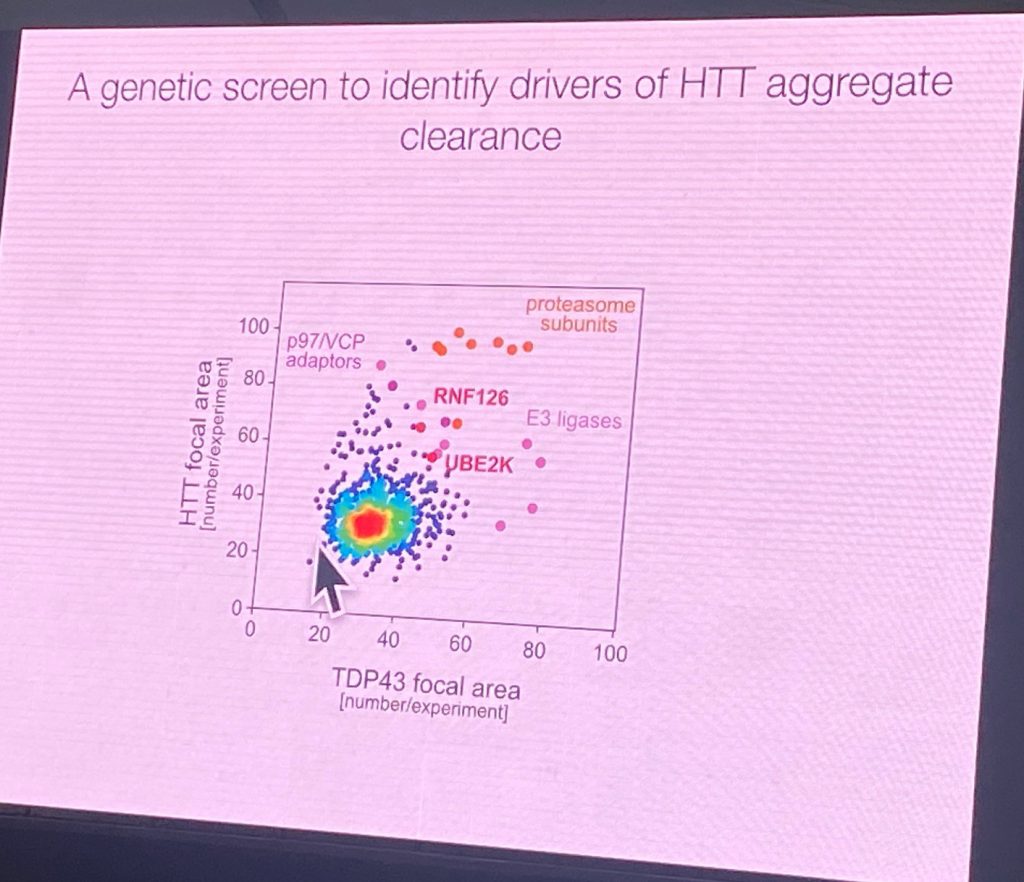
He claims that the degradation signals for HTT are in the region after the first HEAT domain of HTT. Using a GFP fusion with 508 AA N-terminal HTT and performing a screen in stably transfected neuroblastoma cells to identify factors that would promote aggregation of this fragment (which normally does not aggregate). Using various stress signals, aggregates appears in these cells, and after removal of the stressors, the aggregates (‘foci’) are cleared. Extended stress exposure leads to a CAG length dependent aggregation, even after extended washout periods. This enabled an E3 ligase and effectors genomic screen which led to the identification of the complexes that can affect this process, delaying clearance of HTT, RNF126b and UBE2K. RNF126 directly binds to HTT and the p97/VCP disaggregase. It is also highly expressed in the brain. Furthermore, Rape’s lab showed that a degradation assay can be developed to show direct activity of RNF126 complexes on HTT in cells. RNF126 KO increased HTT aggregation, and the over-expression can degrade it in a cellular context. Additionally, a reconstituted ubiquitination system was achieved with purified RNF126 and HTT. Point mutants that inactivated the RNF12 catalytic activity did not have such an activity. The E2 enzymes are UBE2K and UBE2D3. He also showed that this system also works for full length HTT ubiquitination, not just on the shorter reporter system.
His work suggests that a system exists to control HTT aggregation and therefore open the way for the identification of small molecules that can target HTT for degradation.