Revealing mHTT in real-time
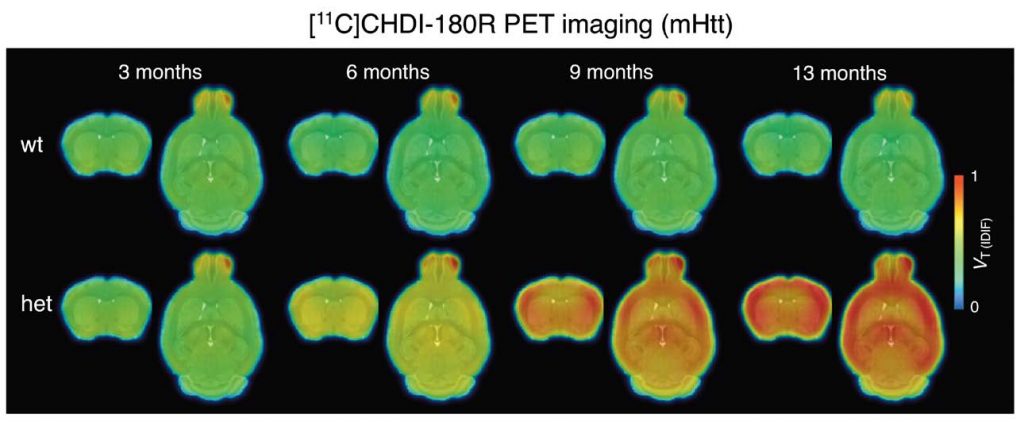
Huntington’s disease (HD) is caused by mutations in the gene encoding for the protein huntingtin (HTT), leading to accumulation of toxic mutant HTT (mHTT) aggregates. Development of therapies aiming to reduce mHTT is hindered by the lack of imaging methods to visualize mHTT in the living brain. Here, Bertoglio et al. developed a positron emission tomography (PET) imaging radioligand that could be used to measure mHTT aggregates in the brain. This probe allowed noninvasive quantification of brain mHTT in a rodent model of HD during disease development and in response to therapeutic intervention, suggesting that PET imaging using this radioligand could be used for improving diagnosis and for measuring the effects of potential therapies.
The work described here started >10 years ago with a collaboration between CHDI Foundation, where our President Ignacio Muñoz-Sanjuan works, and Evotec, a contract research organization with sites in Germany and Cambridge. The work led to the identification of the first ever mutant HTT-specific imaging agent to allow non-invasive monitoring of HTT aggregation in the living brain. In the Science Translational Medicine paper the teams of CHDI, Evotec and University of Antwerp imaging institute demonstrate the utility of this ligand , called CHDI-180R, which is radio labeled with carbon-11, to detect aggregate pathology in living animals, and its use as a biomarker of levels of pathology after therapeutic intervention. The paper effectively demonstrates that this ligand – and others with similar properties – can be used to detect effects on mHTT levels and regional accumulation in a therapeutic context.
This is important because current clinical trials targeting HTT expression can only measure an effect in clinical subjects receiving therapies through the collection of CSF via lumbar ;punctures. CSF mHTT is not informative of the site of action of therapies with a restricted distribution, like the Roche ASOs, or the Uniqure AMT-130 agents. Therefore, the hope is that this agent or others like it, will be used to demonstrate clinical utility in making decisions as to when and where a therapy is lowering mHTT int he human brain and in response to a treatment, enabling better decisions in clinical trials. Of course, detecting mHTT in the human brain might also be informative of understanding progression of the disease.