Gill Bates from University College London, described her work on incomplete splicing of the Htt gene, and the implications of these findings for therapeutic development. The splicing product she identified led to the production of a pure exon-1 protein, which in the context of the CAG expansion, is very toxic. Her recent work has been focused on determining, in the context of knock-in mouse models, whether this abnormal spliced product (which only happens as a function of the expansion) significantly contributes to the disease onset and progression in the commonly used rodent models (and by extension, to the human disease). It is well known that exon-1 is very aggregation prone and therefore this might initiate the disease pathology found in human HD and in mouse models.
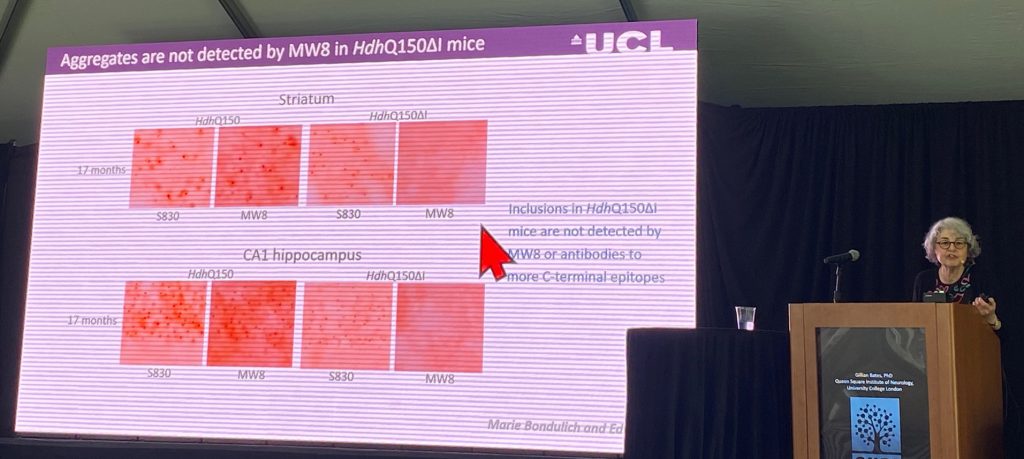
There is some evidence that this abnormal processing can occur in human samples, but the current evidence is stronger for the pediatric HD cases, where the CAG length is larger. This has been shown also in patient fibroblasts and by in situ hybridization (ISH) studies in human nuclei, where her lab can detect expression of this mRNA product. in all mouse models, she can detect the pure exon-1 protein by use of antibody combinations that specifically detect the exon-1 protein (the MW8 antibody is able to only recognize a pure exon-1 protein sequence that ends in a proline residue, and not in the context of the protein generated from the full length mRNA transcript; therefore, MW8 is considered a ‘neo-epitope’ binding antibody).
Gill describes several new mutant HTT antibody based assays to track progression of aggregate pathology in KI mouse models, including monitoring exon-1 aggregation. The aggregation of exon-1 product does not seem to alter full-length HTT levels using standard assays. Using these assays, her lab has now been able to show that in a mouse model where a 20-kb DNA sequence within intron-1 is deleted via crispr editing of a KI model, the HdhQ150 mouse. In this mouse, the exon-1 pure protein product produced via misplacing is reduced by 80-90%, without changing the levels of the full length HTT protein. Importantly, the aggregate pathology is very much diminished (it appears, but very delayed; 3 vs 17-months of age in either model) when the missplicing event is prevented genetically. Additional endpoints like RNAseq and striatal SPN ephors studies are ongoing, but not yet completed.
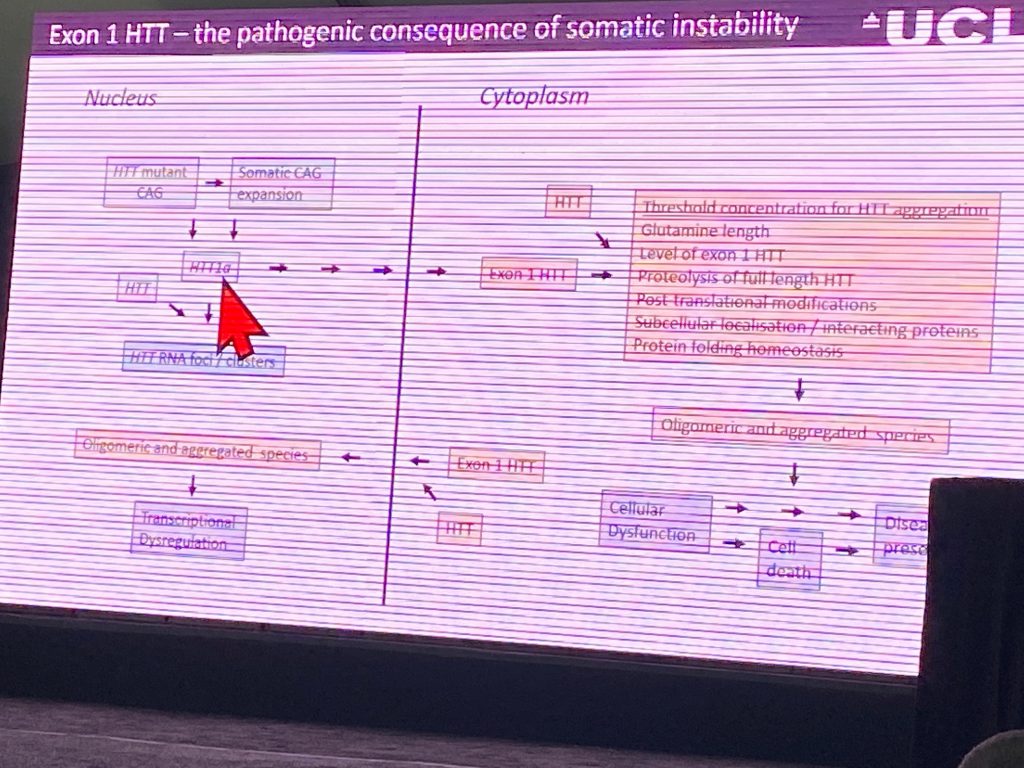
She then proceeded to monitor aggregation driven by the pure exon-1 in humanized YAC128 mouse model, and finds that the earliest and more profound aggregation is found in the cerebellum beginning at 3-months of age. She then switched to using ISH to detect full length HTT transcript vs the exon1a misplaced mRNA, and found them to form ‘inclusions’ or ‘foci’ within the nucleus of neurons which might be pathogenic, as the do not contain normal mouse HTT. These ‘foci’ appear to only occur in a human context, not in the KI mouse models, suggesting that the human intron-1 sequences are likely responsible for the retention of this mRNA in the nucleus. She concluded her talk by summarizing her hypothesis for mechanism of pathogenesis due to missplicing or proteolysis.
Judith Frydman from Stanford University spoke about another aspect of HTT biology implicating translational control of HTT protein levels and the role of mHTT in the stress response in brain cells. Judith here argues for a new mechanism that might contribute to disease pathogenesis, that might explain why in HD there are so many things wrong with brain cells. She is focusing on the role of ribosomal alterations in HD, and the role of HTT in ribosomal biology. The levels and fate of proteins is determined by the rate of elongation of the ribosome. The exon-1 of HTT contains the polyproline repeats, which act as translational stalling sequences, and are very hard to translate, slowing down translation. In addition, HTT has a micro-open reading frame (uORF) in the 5′ UTR of the HTT gene, which is conserved in mammals. uORFs regulate translation and are typically implicated in gene regulation by the cellular stress response. Judith claims that HTT is a ‘stress-response’ gene. She shows that eIF2a-P is an event that regulates HTT translation in response to many potential cues, including at the dendrites and axon terminals.
Using a technique called ‘ribosome profiling’ she shows that both the uORF and the main ORF (encoding HTT) are bound by translating ribosomes, and the extent of occupancy by ribosomes can be modulated by stress signals. HTT expression is induced by stress in various cellular contexts, including primary neurons. The stress bypasses the uORF and shifts translation to the main ORF.
Judith then postulated that this process- the upregulation of HTT via the uORF and stress- could be determining of the protein levels and aggregation process. Therefore, the uORF controls the levels of HTT protein being made.
In an HD context, the collisions are observed in a CAG length dependent manner, by using an artificial system, where viral expression of HTT constructs expressing exon-1. Using puromycin, which induces translational stop, she studies the impact of the CAG repeat on collisions. Collisions can be recognized by a ribosome quality control (RQC) and activates many signaling pathways, as a response to this event, signaling that the cell is having a big problem. Her lab then conducted proteomic studies using the viral polysome system expressing exon-1, and they showed that many E3 ligases are found altered in this system due to the stalling of mHTT, including the depletion of eIF5a, leading to a premature termination of translation.

The eIF5a levels go down in the brains of HD mouse models, and this is supported by ribosome subunit ubiquitination levels, which indicate a ribosome function deficit. In addition, the proteostatic mechanisms are affected early on in the disease process. Judith claims that the loss of eIF5a expression is critical for pathology in HD, via a translation mechanism. She thinks this is a valid therapeutic approach, via the modulation of translational elongation.