Dr. Ignacio Muñoz-Sanjuan, Factor-H president, shares in this article his first insights on the Skyhawk’s Therapeutics announcement from January 28.
There are always two sides with any important development regarding possible treatments for a devastating condition like Huntington’s Disease: one is the scientific part of it; the other is how the results of any trial are communicated both to the clinical & scientific community and to the Huntington’s community at large.
In my experience, information about clinical trials results needs to be communicated in a way that helps manage how the patient community interprets the news, to adequately manage their expectations. In a community so castigated by a terrible disease that affects generations of their loved ones, special care must be taken, and I believe that we, as professionals, must be cautious in communicating the news. I hope I will achieve this goal with this article, even though some parts of it are technical in nature.
A BIT OF BACKGROUND
Last September, uniQure announced the 36-month results of the Phase-2 clinical trial in people symptomatic with HD, with AMT-130, a gene therapy agent. The numbers of individuals enrolled in this long (5 years duration) Phase 2 study is small and the primary endpoint was safety. The results communicated were positive, showing, for the first time, a potential disease stabilization in multiple clinical scales.
At that time, as I wrote about previously (HERE), I really felt that for the first time, there was light at the end of the tunnel; a hope that a therapeutic that silences HTT expression could produce positive clinical benefits. However, I was far from certain about “when” we might definitively achieve this, and what the lasting impact of HTT lowering drugs would be in the multifaceted clinical symptoms patients experience.
That September morning my email and WhatsApp inbox was full of text messages from people from several countries around the world, including places where I knew the therapy, if approved, might not be available for years to come, if at all. As a scientist, I know results always come with caveats and are subject to interpretation; there are no certainties in research. The main “issue” here was not the results of the study. The main issue but was how the potential caveats of the study – such as a small sample size, potential placebo effects, the use of an external natural history control group, might alter the interpretation of the results. Unfortunately, the mass media portrayal of the results led the community to believe that a ‘cure’ had been found, creating an erroneous sense in the worldwide community that our work was ‘done’.
The impact of these news in the patient community was not that positive. People reacted in ways that, surely, were not anticipated by the medical professionals – they thought a “cure” was ready, was ready now. This false sense of expectation led many concerned families to feel despair, not hope. Despair at not knowing how to access the treatment, and whether it was too late for their loved ones. Young people at risk questioned whether they should now get tested, so they could access a therapy that was not even yet approved.
A NEW ANNOUNCEMENT, GOOD NEWS WE NEED TO UNDERSTAND IN MORE DEPTH
On Jan 28, 2026, Skyhawk Therapeutics released the nine-month interim results from the Phase 1c portion of the clinical trial called “Falcon-HD”, evaluating the experimental agent called “SKY-0515” for the treatment of Huntington’s disease (HD). Phase 1c studies are the first studies conducted with an experimental therapy in the patient population, mainly to ensure tolerability, usually in a small number of patients.
This early-stage trial is primarily designed to assess safety and tolerability, but it also provides initial biological and exploratory clinical signals that are of considerable interest to the HD community. Importantly, please remember that the study has not yet been completed. You can read the official SKY-0515 press release HERE
TRIAL DESING AND PATIENT POPULATION
The Phase 1c study enrolled individuals with early manifest Huntington’s disease in stages 2 and mild stage 3 in what we call the “Integrated Staging System, or ISS”. Participants received either placebo or one of two oral doses of SKY-0515 (3 mg or 9 mg) for three months, followed by a nine-month “open-label” extension (OLE). Patients in the placebo group during the first 3 months of the study could elect to switch to the active drug, therefore the study becomes ‘unblinded’. Both the patients and the doctors now know all the patients in the open label extension are taking the drug, and therefore there is no placebo control group after this period. The interim analysis reported by Skyhawk reflects data from the first nine months of treatment, covering the placebo-controlled period and the initial six months of the OLE.
The Phase 1c trial is being conducted in Australia and New Zealand, and enrollment numbers are necessarily small, as is typical for Phase 1c studies.
MECHANISM OF ACTION: LOWERING HUNTINTIN VIA SPLICING MODULATION
SKY-0515 is an orally administered small-molecule drug (in a pill) that targets the huntingtin (HTT) transcript via the modulation of a molecular process called ‘pre-mRNA splicing’, a natural process in every cell that works to process the nascent messenger RNA into its mature form, leading to the synthesis of proteins. The drug alters the splicing of the HTT pre-mRNA by promoting retention of intron-49, a sequence that normally would be removed during mRNA processing. Because intron-49 contains premature stop codons, its retention leads to early termination of translation and subsequent nonsense-mediated decay of the full-length HTT mRNA. The sequences in intron-49 in the HTT gene do not differ between the mutant (expanded CAG repeat, located in exon-1 of the gene) and the unexpanded copy, and as a result, SKY-0515 reduces the levels of both mutant and normal full-length HTT proteins. This is called a ‘non-selective’ lowering therapy, because both copies of the HTT mRNA get degraded.
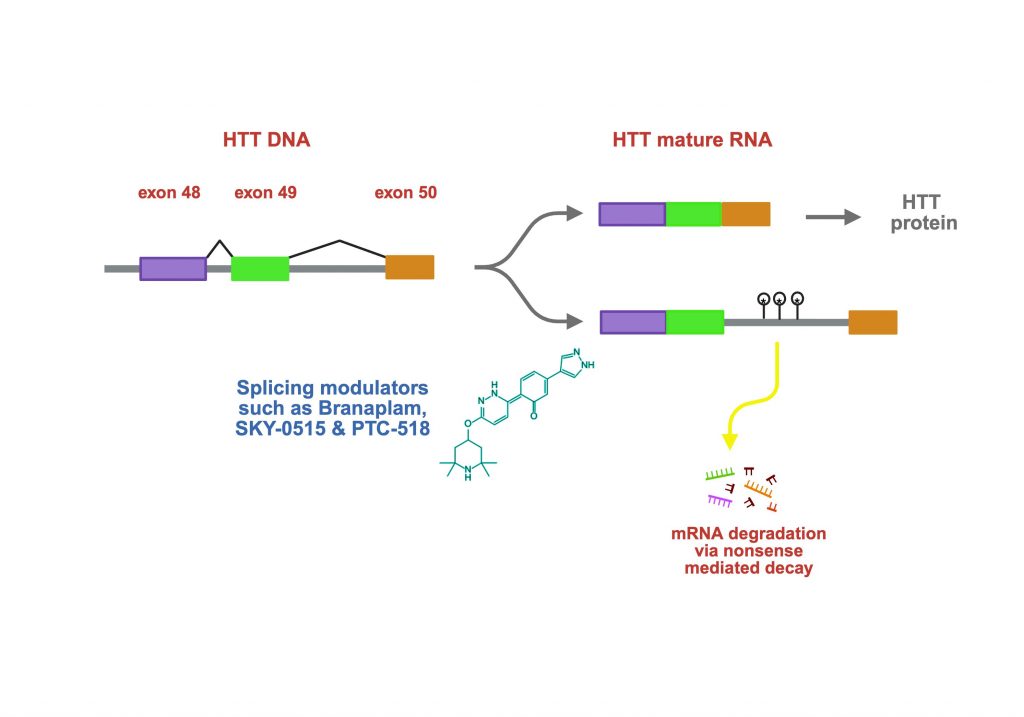
Notably, this approach does not reduce the shorter exon-1–derived HTT fragment (often referred to as exon1a), which has been implicated in nuclear aggregation and toxicity in HD.
This distinguishes SKY-0515—and similarly acting splicing modulators such as PTC-518/votoplam from PTC Therapeutics/Novartis—from RNA-interference approaches like uniQure’s AMT-130, which directly target exon 1 and lower both full-length and exon-1 HTT mRNAs, lowering the proteins they encode.
Another practical distinction is delivery: SKY-0515 is taken orally and distributes systemically throughout the entire body including the entire brain, whereas AMT-130 requires a neurosurgical procedure to deliver the therapeutic agent directly into the striatum.
BIOMARKER EFFECTS: STRONG PERIPHERAL mHTT LOWERING, LIMITED CNS DATA
In the press release, Skyhawk reported a robust reduction of mutant HTT (mHTT) protein levels in blood cells. At the highest dose tested (9 mg), mHTT levels were reduced to approximately 62% of baseline levels, representing the largest blood-based mHTT lowering reported to date for a splicing modulator. This represents evidence that the drug is working as expected, another key goal of the Phase 1c study.
However, despite statements indicating good brain penetration, the company did not disclose quantitative data on mHTT lowering in cerebrospinal fluid (CSF), which is a “proxy” for brain lowering of HTT. Given the central role of CSF biomarkers in assessing brain pharmacological activity, this omission leaves an important gap in the interpretation of central efficacy at this stage.
In addition to targeting HTT levels, SKY-0515 also lowers the expression of the PMS1 protein, a protein identified in genome-wide association studies (GWAS) for genes whose function or expression might alter the rate of disease progression in HD. PMS1 is known to modulate the somatic instability of the CAG repeat in the HTT gene in neurons of the brain, potentially explaining the effects of genetic variants in PMS1 in changing the early stages of HD disease progression. The level of PMS1 reduction observed with SKY-0515 in blood is 26%, probably reflecting a lower potency of SKY-0515 against this gene, as compared to HTT.
It is unclear if at the doses administered, the drug is indeed capable to lowering PMS1 in the brains of HD patients. No information has yet been disclosed about the potency difference against these 2 targets and the predicted exposures needed to lower PMS1 in brain sufficiently to modulate somatic instability of the CAG repeat.
SAFETY AND TOLERABILITY
From a safety standpoint, SKY-0515 appears to be well tolerated after nine months of dosing (as described by Skyhawk, 90 patients have now received the drug), suggesting that the primary endpoint of the Phase 1c study has been met. There was no evidence of ventricular enlargement, a neuroimaging signal associated with adverse outcomes and other biomarker changes, such as those reported in Roche’s ASO tominersen Phase 3 trial. This is interesting as well given the recent reports at the 2025 HSG (Huntington Study Group) annual meeting, where Novartis disclosed ventricular changes with votoplam, another splicing modulator oral drug.
That said, other commonly monitored markers of neuronal injury and inflammation, such as neurofilament light chain (NfL) levels in CSF, have not been disclosed. The absence of reported safety concerns is reassuring, but fuller biomarker data will be important as development progresses.
EXPLORATORY CLINICAL SIGNALS: UNEXPECTED IMPROVEMENT OF cUHDRS SCORES
Perhaps the most striking aspect of the interim report is the exploratory analysis of clinical outcomes. Skyhawk presented changes in the composite Unified Huntington’s Disease Rating Scale (cUHDRS), comparing treated patients to an externally matched natural-history cohort drawn from Enroll-HD and TRACK-HD.
In this analysis, both dose groups were pooled (17 treated participants total) and compared to a large external control group (n ≈ 325) pooled from the Enroll-HD and TRACK-HD longitudinal natural history studies. The details of the characteristics of the matching group have also not been disclosed. Over the nine-month period, patients receiving SKY-0515 showed an average improvement of +0.64 points on the cUHDRS, whereas the external control group declined by −0.72 points. The magnitude of this difference is approaching the difference in the cUHDRS reported by Uniqure’s AMT-130 Phase 2 studies after 36 months.
To my knowledge, this represents the first report of a numerical improvement, rather than stabilization, in the cUHDRS over this time frame in an interventional HD study. Notably, the divergence between treated patients and controls appeared early—within three months—and continued throughout the observation period.
INTERPRETING CLINICAL MEANINGFULNESS
There has been extensive debate in the field about what constitutes a clinically meaningful change in the commonly used HD scales in clinical trials. Recently, Professor Wild wrote a nice piece about this very topic; you can read it HERE
While caution is clearly warranted—given the small sample size, open-label design, and reliance on external controls—the magnitude of the observed difference is approaching thresholds often considered clinically relevant.
It would have been particularly informative to see results broken down by individual UHDRS subscales (motor, cognitive, and functional measures). Among these, the Total Functional Capacity (TFC) score is especially valuable, as it is less susceptible to placebo effects and more directly reflects day-to-day functioning.
WHAT COMES NEXT—AND WHY THIS MATTERS
Overall, these early data are encouraging for both the trial and the broader HD community. Skyhawk has now initiated a Phase 2/3 study (which might lead to potential approval of this therapy if indeed it improves disease progression) of SKY-0515 in Australia and New Zealand, with additional sites expected to open soon. Completion of the full 12-month Phase 1c plus OLE dataset will be an important next milestone.
Beyond the implications of these results for this program, these results raise broader questions for the field:
Is systemic HTT lowering well tolerated in humans?
Thus far, based on the collective results from the uniQure, Skyhawk and Novartis trials, the answer appears to be yes, though understanding the extent of HTT reduction achieved in different tissues—particularly throughout the brain—will be critical.
Is selective targeting of exon-1 HTT necessary for clinical benefit?
The apparent clinical improvement reported here suggests that robust lowering of full-length HTT alone may be sufficient, challenging a long-standing and actively debated hypothesis in HD biology. However, these studies are still early and we need to await further clinical results.
A FIELD AT AN INFLECTION POINT
HTT-lowering strategies have been pursued in earnest since 2006, when an antisense oligonucleotide program first entered the field after the CHDI-Ionis collaboration began. After years of setbacks and hard-earned lessons, multiple approaches—including splicing modulators, ASO- and gene-therapy–based strategies—are now demonstrating acceptable safety profiles in symptomatic patients (not to be taken for granted), along with potentially meaningful early signals of benefit.
Both programs discussed here suffer from similar criticisms – low sample size, the use of natural history external controls, OLE, and potential placebo effects. The key difference is the speed at which a small molecule agent can be advanced through rigorous Phase 2-3 trials, which will include a suitably powered placebo control group in the case of the Falcon-HD study.
While the effects reported by Skyhawk remain modest and must be interpreted cautiously, the overall trajectory is encouraging. The field appears to be entering a phase of greater maturity, with multiple independent lines of evidence converging on the feasibility of HTT-targeted therapies.
It is an exciting—and hopeful—moment for patients, families, and researchers alike.
Thank you for reading.
Ignacio Muñoz-Sanjuan, Factor-H President



