Jim Rosinski
Jim Rosinski from the CHDI Foundation spoke about the -omics strategy at CHDI Foundation to identify early biomarkers of disease progression, with a focus today on the early analysis of the CSF and plasma samples derived from the HD Clarity study using the Somalogics platform. The goal of the strategy is to apply mutilple platforms to identify/align biomarkers of disease progression that can be turned into validated, quantitative, assays to use in clinical programs. The Enroll-HD platform, given its size and longitudinal nature, forms the basis for the entire -omics strategy. Jim explained some of the current technology platforms being deployed for analysis of blood and CSF, including mass-spec based detection, small RNA analysis, Somalogic proteomics platforms, and epigenetic, methylation, analysis of blood cells, among others.
A collection 579 CSF and serum samples were analyzed from the HD Clarity study (420 participants, 150 repeated samples fro replication) using the aptamer (DNA)-based Somalogic platform (7629 proteins being measured, although HTT is not on their platform). Jim described the preliminary, initial analysis of these samples using this technology. The analysis of the “repeated visits” (1-month apart) showed a very good correlation (r=0.7-0.85) detecting about 3500 proteins in CSF. Analysis was done by Doug Langbehn from University of Iowa.
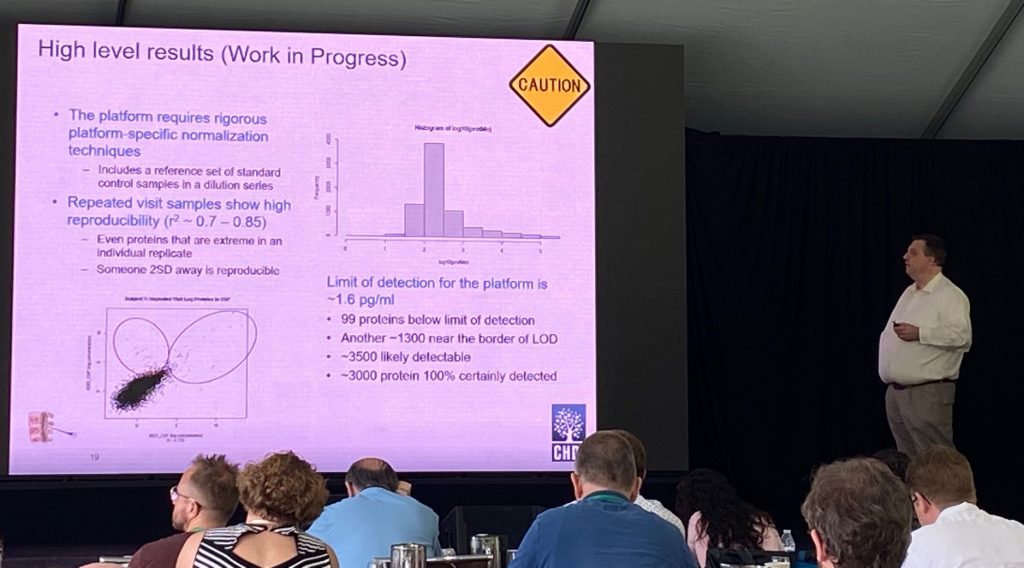
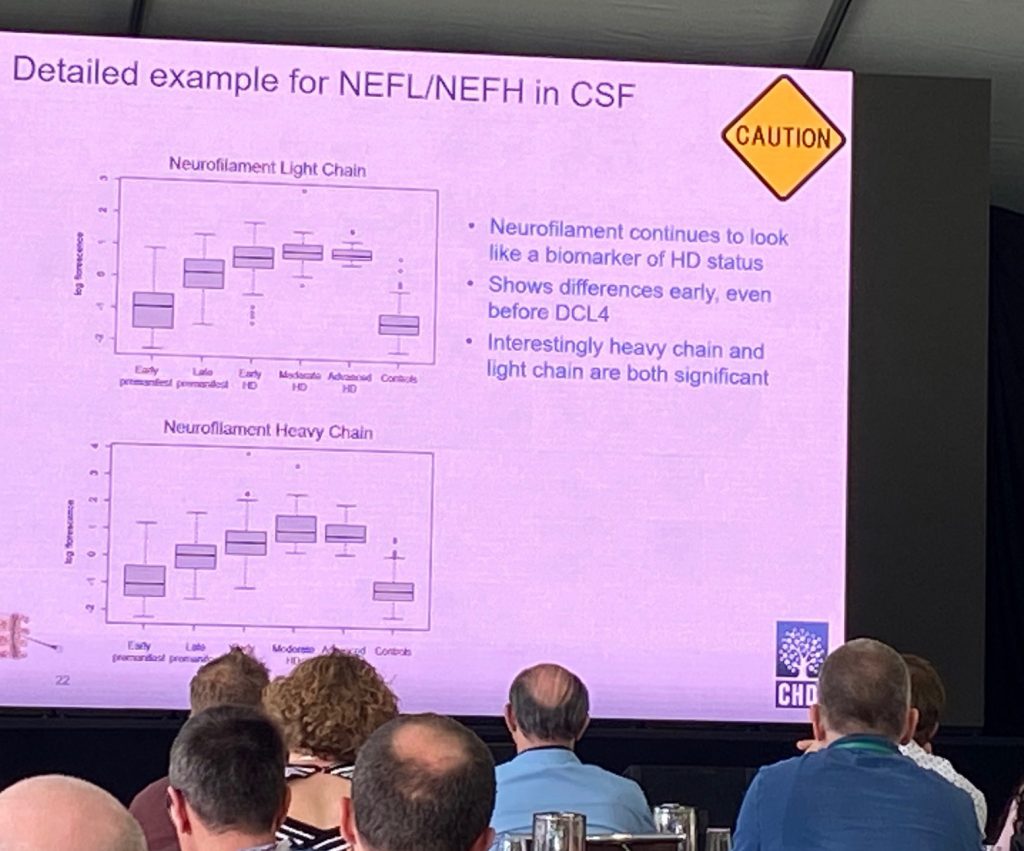
Initial data analyses showed that there are a good number of proteins (97) being different in CSF and serum with FDR<0.05, most notably neurofilament (NF) proteins NF-light and NF-heavy chains. Both show significant differences from early pre-manifest. Indeed, these two proteins NEFL/NEFH, can predict genetic status of the participants, and are highly correlated with CAP scores and cUHDRS. An additional protein of interest of CNP (2′, 3′-cyclic nucleotide 3′ phosphodiesterase), an enzyme mostly expressed in oligodendrocytes, and might be a good marker of demyelination or axonal degeneration. The enrichment analysis of the CSF proteome altered in these samples show that the signature is an HD signature, commonly shared with HD mouse models including Q175 and R6.2 proteomic/genetic changes. The serum changes are less impressive, but there is a still a signature that might predict cUHDRS control using just 5 proteins.
Aline Delva
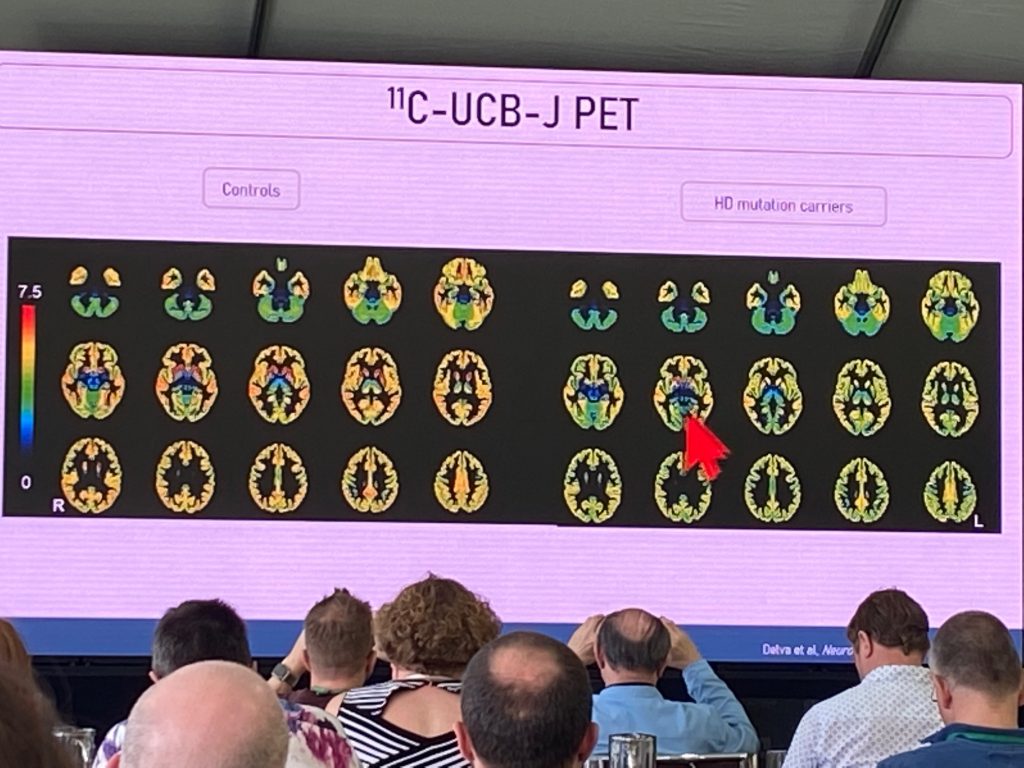
Aline Delva from Leuven University, spoke about the first evaluation of a mutant HTT imaging tracer [C11] -CHDI’180R, developed by CHDI Foundation, in HD patients. In addition, she described the work conducted to image presynaptic terminal changes using the Sv2a PET tracer [C11] UCB-J, in early HD individuals. She started first with a description of early synapse loss in HD post-mortem tissues and in HD models. The use of this novel tracer can be indicative of degenerative changes in the brain of people at various stages of disease, and can also act as a marker of synaptic regenerative or neuroprotective strategies. Aline also included [F18]FDG-PET in an initial study to compare synaptic density changes with functional activity, and with structural MRI. In a recently published study, a total of n=18 HD carriers (n=7 premanifest and n=11 manifest) and n=15 controls, were included.
The FDG-PET changes correlate well with UCB-J changes throughout the brain. The hypo metabolism signatures replicate prior changes, although the UCB-J signal suggests widespread changes in synaptic density in frontal-temporal-parietal cortex, basal ganglia and cerebellum, evident already in the premanifest stage. UCB-J was most sensitive marker in premanifest stages, and correlates extremely well with UHDRS motor score and SDMT task. The motor score was best correlated with UCB-J binding changes in putamen. This is a very significant marker and suggests strongly that early HD involves significant presynaptic changes, beginning in the striatum (probably afferent fibers coming from cortical or thalamic regions), and then progressing to extra-striatal regions.
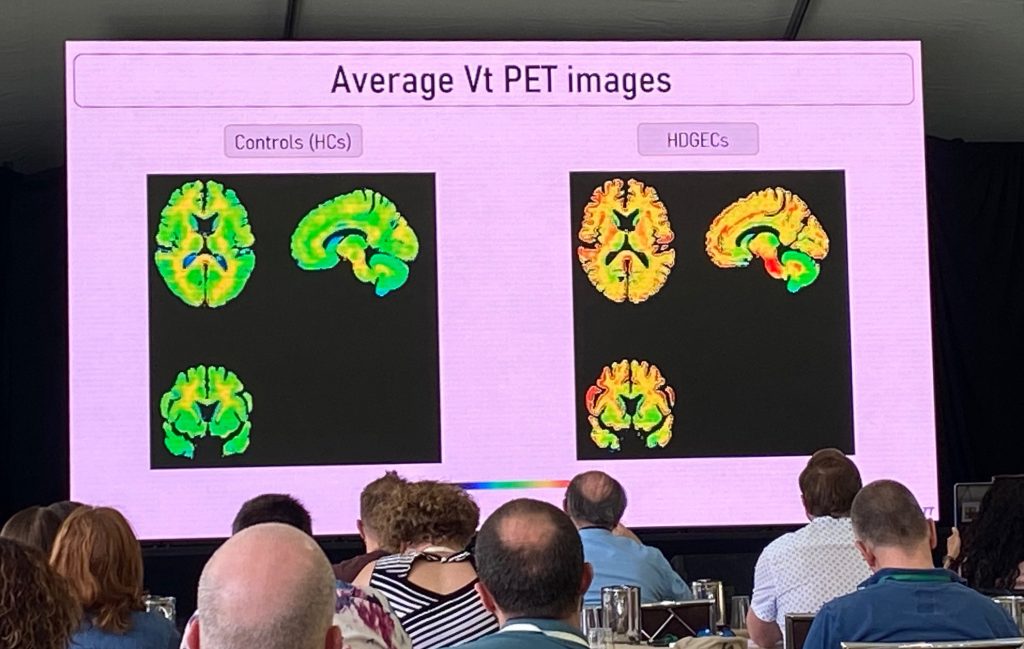
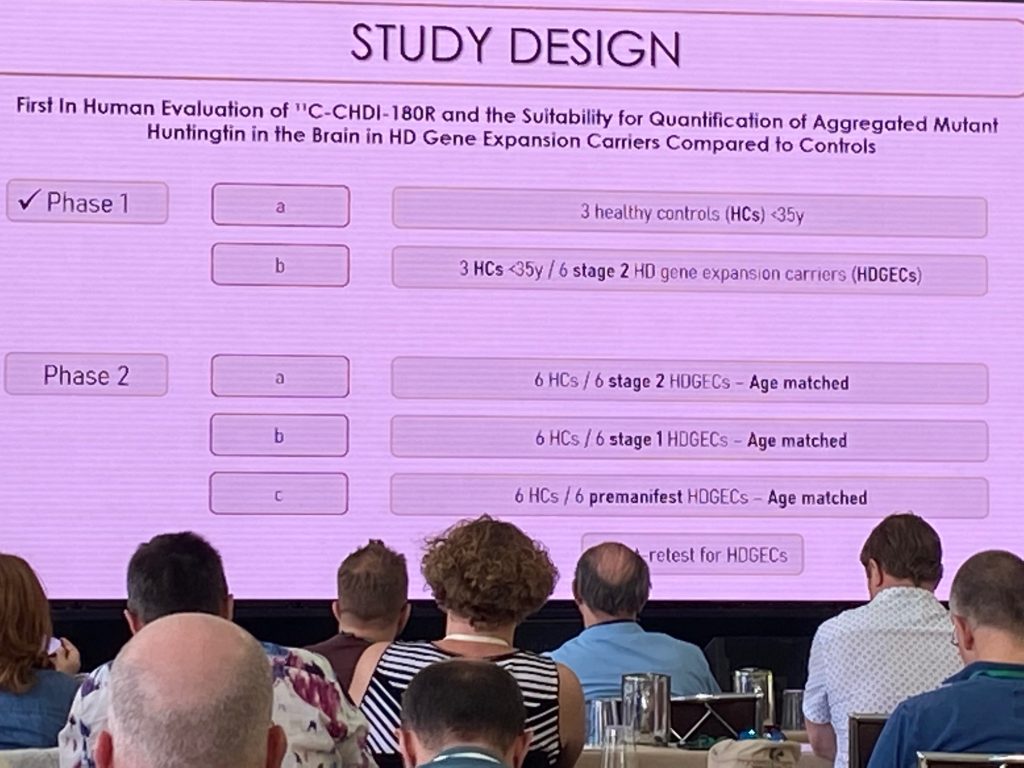
Next, Aline describes the first in-human work conducted with the novel mHTT specific PET imaging tracers, in a collaboration with CHDI Foundation. She summarized the prior preclinical work conducted by CHDI, and the results of the human dosimetry studies, which showed that [C11] -CHDI’180R could advance to HD clinical studies due to their safety, brain kinetic and metabolic profiles.
The ImageHTT study is the first to evaluate differences in [C11] -CHDI’180R binding to the HD brain vs control brains in the first study of its kind. The phase 1 of this study results were discussed, where a few young controls vs stage 1-2 HD patients (n=6 per group) were evaluated. Additional studies involving more HD individuals and better matched for age will be included, as depicted below.
The mean volume of distribution (Vt) is higher in the HD vs controls, although there is considerable variability across the various signals, and therefore the overall group differences were not significant. Using a different technique (mean DVR, distribution volume ratios), statistical significance was obtained for some cortical regions, although the effects are small. More work is needed (test-retest reliability, more subjects and different stages of HD), but the initial findings are encouraging. There are additional new-radioligands are coming up behind, such h as [C11] -CHDI’009, which has a much higher binding in preclinical studies.