Beth Stevens
Beth Stevens from HHMI and the Broad Institute spoke about her work in protecting synapses in the context of HD and the role of the immune system and microglial cells of the brain in modulating synaptic pruning in the adult brain, which enables the ‘sculpting’ of synaptic circuits. The hypothesis is that aberrant microglia activity via the complement system leads to excessive synaptic elimination, which contributes to alterations in HD pathogenesis. Microglial activation is a hallmark of brain damage and their reactivity if highly context dependent, leading to beneficial and detrimental effects of their activation, for example through cytokine release.
A critical role Beth studies is the modulation of synapses by microglia, both during development and in disease. The mechanism by which these cells modulate synaptic number is through the signaling pathway called the ‘classical complement cascade’, including C1q, C3 and C4 proteins. Microglia express complement receptors (C3R) and these are essential for microglial function. Phosphatidyl serine (PS) is a lipid highly expressed in brain cells and that acts as a critical regulator of the complement system. A set of protective molecules (“don’t eat me” signals) such as CD47-Sirpa are important mediators in microglial pruning in synapses. Her model includes aberrant activation of microglial synapse-mediated elimination early on in neurodegenerative diseases such as AD, MS and HD, in a region- and synapse-specific way.
The work her lab has done in HD involves studies in mouse models of HD (BAC-HD and Q175) and in assessing levels of complement proteins in CSF during disease progression, which has led the way for a company, Annexon, to develop a blocking monoclonal antibody to C1q protein. She also worked with human tissue samples to assess the excessive deposition of activated complement proteins in vulnerable synapses in HD. In mouse models of HD, she showed evidence of synaptic loss (Homer and vGlut1 proteins). This was mirrored by an enhanced deposition of C1q and C3 in vulnerable synapses in the striatum. We describes that cortical afferent synapses (Vglut1 expressing) but not thalamic afferents (Vglut2 expressing) are affected. These observations appear to be present too in HD human tissue samples.
In Q175 mice, microglia were shown to engulf more cortico-striatal projections, through studies were they tagged specific cortical projections with GFP and monitoring the activity of microglia in phagocytosis of afferent cortical axons. Based on this work, Annexon developed a C1q blocking antibody, which inhibits the activation of the complement cascade. The antibody is delivered IV and they claim that this antibody crosses the BBB. When Q175 mice are dosed with the antibody for 1 month, synapse loss is reduced, complement activation is reduced, and also a partial restoration of SPN synapse activity. This work has been followed in genetic crosses between the BACHD and a C3 KO model – which also supports the notion that inhibiting this pathway is neuroprotective in this model.

Another strategy her lab employed was to target the receptor for complement (C3R) by knocking out the receptor in microglia, also leading to protection of the synapse loss in the striatum. They extended these initial studies to show improvement of a deficit in a visual discrimination task when C3R is genetically deleted. Finally, Beth described the work ongoing to measure levels of complement proteins in CSF and plasma of HD individuals vs controls, using the Clarity cohort. Sample sizes are small (15-20 per group in early HD vs 32 control samples) but show an early increase in C3 and C3b (activated C3) levels in early disease. C4 levels have not yet been measured and C1q levels did not change.
Leslie Thompson
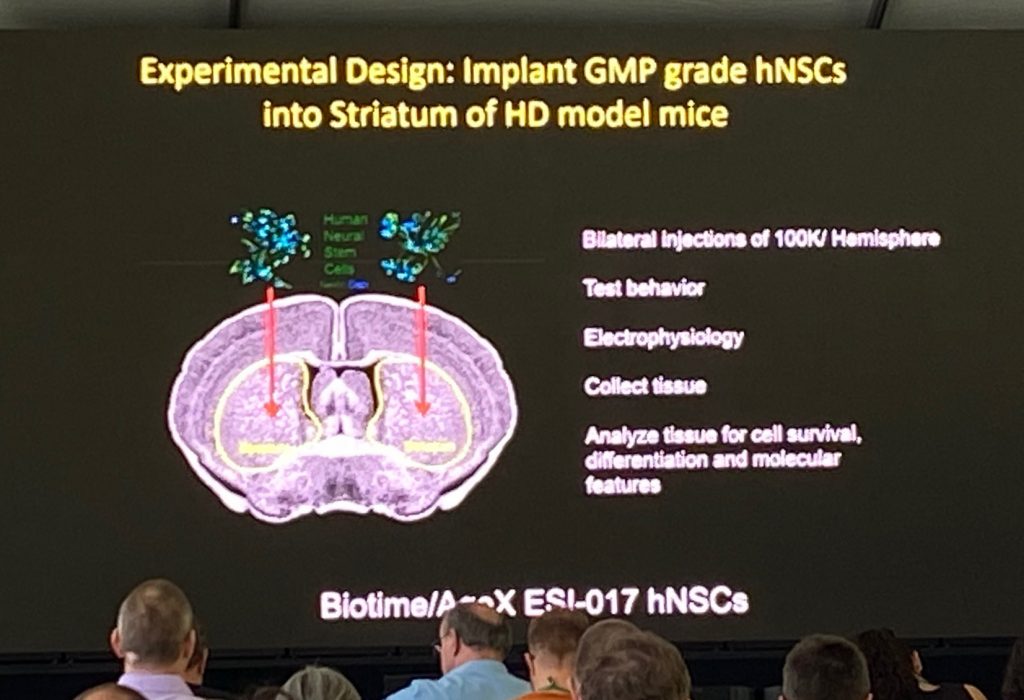
Dr. Leslie Thompson from University of California, Irvine, spoke about her work leading a human neural stem cell program (find out more in the following link for SC4HD) for the treatment of HD, whose aim is to restore affected circuits when these cells are transplanted into the striatum of patients. Leslie described the group’s activities, including REPAIR-HD work with implanted MSNs and that of SANA and Steve Goldman in the glial progenitor transplantations. Her major work focuses on production and transplantations of ESI-017 human neural set cells produced under GMP conditions. She described the QC of the neural nature of the cells by scRNAseq and marker analysis to ensure that no pluripotent stem cells are present in the clinical preparations (pluripotent stem cells can be tumorigenic if grafted).
Leslie described the work with transplantation studies of ESI-017 derived neural line in R6/2 and Q175 mice, which have shown significant beneficial effects in these models, including in aggregation pathology and behavioral alterations. The mechanisms by which these effects are obtained is unclear, and Leslie described the potential effect of BDNF expression in the grafted cells. The expression if BDNF is being used in a “potency assay” (an assay that allows medical and regulatory agencies to evaluate whether the same ‘grade of the cells’ is being used) – a requirement for clinical studies using stem cell transplantation therapeutic development. single cell analysis of transplanted cells after 8 months in vivo was shown to contain 3 different cells types, although the majority was neuronal (mix of GABAergic and Glutamatergic cells). However, the grafted cells contain a mix of different populations.
She concluded her seminar by discussing next steps to a therapeutic trial.